Reading time: 18 minutes
Is your manufacturing operation struggling to balance innovation with compliance? You're not alone. 73% of manufacturers report that maintaining ISO certification while implementing new technologies is their biggest operational challenge—yet those who succeed see dramatic results, including up to 92% reduction in documentation errors and significantly improved market access.
In this comprehensive guide, you'll learn:
- How ISO 9001:2015 enables rather than hinders technology adoption
- Step-by-step processes for implementing AI, IoT, and Industry 4.0 technologies while maintaining certification
- Specific compliance requirements for digital transformation initiatives
- Real-world case studies from manufacturers who got it right
- How to prepare for the 2026 ISO 9001 revision addressing Industry 4.0
Whether you're implementing cloud-based quality management systems, IoT sensors, AI-powered inspection, or digital twins, this guide provides the roadmap for maintaining ISO compliance throughout your digital transformation journey.
Table of Contents
- Why ISO Compliance Enables Technology Innovation
- ISO 9001:2015 – Your Foundation for Quality & Innovation
- Essential ISO Standards for Manufacturing Technology Implementation
- 5-Step Process for ISO-Compliant Technology Implementation
- Digital Transformation: Specific ISO Considerations
- Overcoming Common ISO Compliance Challenges
- Real-World Success Stories
- Preparing for ISO Audits with New Technology
- Future-Proofing Your Quality Management System
- Frequently Asked Questions
- Conclusion: Your Next Steps
Why ISO Compliance Enables Technology Innovation
Manufacturing has evolved from isolated production environments to interconnected ecosystems where quality, security, and sustainability must coexist with technological advancement. Far from being bureaucratic hurdles, ISO standards have transformed into dynamic frameworks that actually accelerate innovation when properly understood and implemented.
The Strategic Value of ISO Certification in Modern Manufacturing
Today's manufacturing environment demands seamless interoperability between quality management systems and emerging technologies like artificial intelligence, Internet of Things devices, and digital twins. Companies that successfully integrate these technologies with ISO frameworks are seeing transformative results.
The data tells a compelling story:
- 92% reduction in manual documentation errors when IoT-enabled production lines automatically log deviations into quality management systems
- 37% reduction in physical prototype testing with digital twin technology while maintaining full regulatory compliance
- 70% faster document update cycles with cloud-based quality management systems
Key Insight: ISO-certified facilities that embrace digital transformation aren't just maintaining compliance—they're gaining competitive advantages. Global supply chains increasingly prioritize ISO-certified partners, with most nearshoring agreements now requiring real-time ISO compliance monitoring via digital QMS platforms.
How ISO Standards Support Innovation
Rather than hindering innovation, properly implemented ISO standards accelerate technology adoption by providing structured frameworks for managing change. The risk-based approach of modern ISO standards encourages manufacturers to systematically identify, assess, and mitigate risks associated with new technologies—reducing implementation failures and costly setbacks.
Leading manufacturers leverage ISO frameworks to create technology roadmaps that align innovation initiatives with compliance requirements. By incorporating compliance considerations early in the technology adoption process, these companies avoid the common pitfall of retrofitting compliance onto already-implemented systems—an approach that typically leads to higher costs and implementation delays.
“ISO accreditation serves as a great marketing tool, but its real power comes from how it enables manufacturers to systematically incorporate new technologies while maintaining quality and compliance across their operations.” — Manufacturing Quality Expert
The documentation requirements of ISO standards, often viewed as burdensome, actually provide valuable structure for technology implementations. When manufacturers maintain comprehensive records of technology specifications, risk assessments, validation procedures, and training programs, they create institutional knowledge that supports both compliance and continuous improvement.
Need help navigating ISO requirements during your digital transformation? MSI's ISO Consulting Services provide expert guidance for manufacturers implementing new technologies while maintaining certification.
ISO 9001:2015 – Your Foundation for Quality & Innovation
ISO 9001:2015 serves as the cornerstone for quality management in manufacturing operations worldwide. This foundational standard has evolved significantly since its inception, now providing an adaptive framework that can accommodate cutting-edge technologies while maintaining its core quality principles.
What Makes ISO 9001:2015 Technology-Ready
The 2015 revision introduced a stronger focus on risk-based thinking and organizational context, making it particularly relevant for manufacturers introducing new technologies. Rather than prescribing specific methods, ISO 9001:2015 emphasizes outcomes and performance—giving manufacturers flexibility to leverage modern technologies while meeting quality objectives.
Key clauses for technology adoption:
- Clause 4.4 (Process Management): Defines how processes should be controlled and improved
- Clause 6.1 (Risk Management): Establishes risk-based thinking framework
- Clause 7.1.3 (Infrastructure): Addresses equipment and technology requirements
- Clause 8.5.1 (Controlled Production): Governs production and service provision controls
These clauses provide the framework for ensuring that innovations enhance rather than compromise quality. The standard's process approach is particularly valuable for identifying how new technologies impact existing quality processes and where adjustments may be needed.
Preparing for ISO 9001:2026 and Industry 4.0
The upcoming 2026 revision of ISO 9001 promises to address Industry 4.0 technologies head-on, providing explicit guidance on integrating AI, IoT, blockchain, and other digital technologies into quality management systems. Forward-thinking manufacturers are already preparing for these changes by building more flexible quality management frameworks that can adapt to both current and future technological innovations.
What to expect in the 2026 revision:
- Explicit guidance on AI governance and validation
- Requirements for cybersecurity integration with quality systems
- Digital documentation and electronic signature protocols
- Cloud computing and data integrity standards
- Automated data collection and IoT device controls
Don't wait for 2026 to start preparing. Manufacturers implementing technology governance frameworks now will find themselves ahead of the curve when new requirements take effect. Learn more about preparing for ISO 9001:2026.
Essential ISO Standards for Manufacturing Technology Implementation
Navigating technology adoption in manufacturing requires familiarity with several key ISO standards, each addressing different aspects of operations. While ISO 9001 provides the overall quality framework, other standards address specific concerns related to information security, environmental impact, worker safety, and laboratory testing—all of which may be affected by new technologies.
The interconnected nature of these standards creates a comprehensive system for managing technology-related risks across all aspects of manufacturing operations. Forward-thinking manufacturers recognize that siloed compliance approaches are ineffective in today's integrated manufacturing environments, instead opting for unified management systems that address all applicable standards simultaneously.
Critical ISO Standards for Technology Adoption
ISO 9001:2015 – Quality Management Systems Provides the core quality management framework applicable to all manufacturing operations. Essential for ensuring new technologies enhance product quality and process consistency. Learn more about ISO 9001 certification →
ISO 27001 – Information Security Management As manufacturing operations become increasingly digitized, ISO 27001 has evolved from a nice-to-have to an essential standard for technology-driven factories. This standard addresses the critical security implications of connected manufacturing systems, providing frameworks for identifying information assets, assessing security risks, and implementing appropriate controls.
For manufacturers implementing IoT devices, cloud-based systems, or integrated supply chain platforms, ISO 27001 compliance helps protect sensitive production data and intellectual property while preventing operational disruptions from cybersecurity incidents.
ISO 14001 – Environmental Management Addresses environmental aspects of new technologies, increasingly important for sustainability initiatives. New manufacturing technologies often have environmental implications—from energy consumption of data centers to disposal requirements for electronic components—that must be managed within your environmental management system. Review ISO 14001 requirements →
ISO 45001 – Occupational Health & Safety Covers worker safety considerations for automated systems and new manufacturing technologies. As robots, cobots, and automated systems become more prevalent, ISO 45001 provides the framework for ensuring these technologies enhance rather than compromise worker safety.
ISO/IEC 17025 – Testing and Calibration Laboratories Critical for manufacturers with testing facilities implementing advanced measurement technologies. This standard ensures that sophisticated testing equipment and automated measurement systems maintain accuracy and traceability to recognized standards.
Understanding Integrated Management Systems
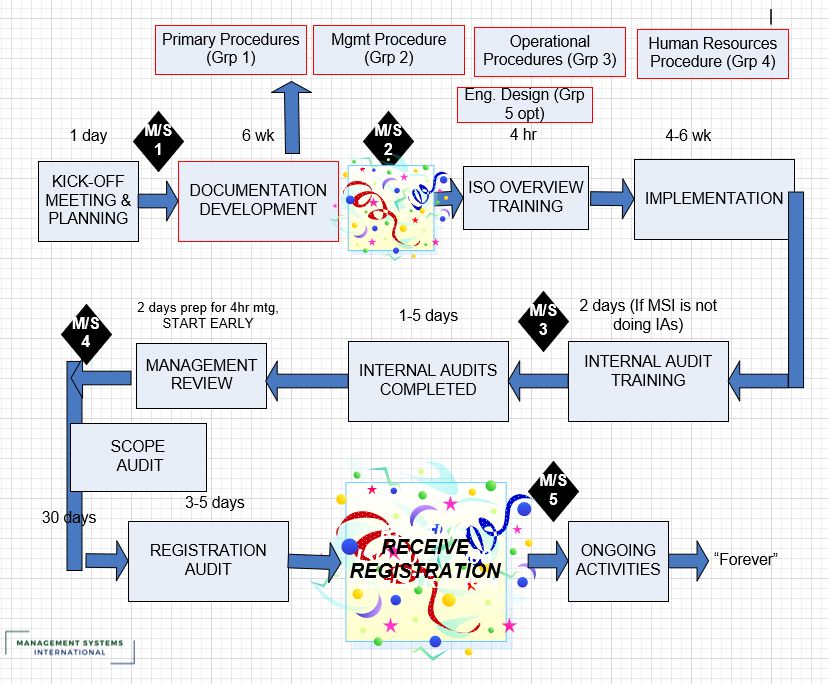
Rather than managing each standard separately, leading manufacturers implement integrated management systems (IMS) that address multiple ISO requirements cohesively. This approach recognizes that modern technologies impact quality, security, environmental, and safety considerations simultaneously.
Benefits of integrated management systems:
- Reduced duplication of documentation and procedures
- More efficient audit processes (combined surveillance audits)
- Better alignment between different management system requirements
- Streamlined technology implementation across multiple compliance domains
- Lower overall compliance costs
5-Step Process for ISO-Compliant Technology Implementation
Successfully implementing new manufacturing technologies while maintaining ISO certification requires a systematic approach that integrates compliance considerations throughout the adoption process. This five-step framework has been proven across hundreds of successful technology implementations in ISO-certified facilities.
Step 1: Conduct Comprehensive Risk Assessment
Before implementing any new technology, manufacturers must conduct thorough risk assessments that identify potential impacts on product quality, process performance, data security, worker safety, and environmental compliance. This risk-based approach aligns directly with ISO 9001:2015's emphasis on risk-based thinking.
Your risk assessment should address:
- Quality risks: How might the technology affect product conformity?
- Security risks: What cybersecurity vulnerabilities does the technology introduce?
- Process risks: Could the technology disrupt existing validated processes?
- Compliance risks: Does the technology create new regulatory obligations?
- Operational risks: What happens if the technology fails or performs unexpectedly?
Document your risk assessment thoroughly, including the methodology used, risks identified, probability and severity ratings, and mitigation strategies for significant risks. This documentation becomes essential evidence during ISO audits that you've implemented appropriate controls before deploying new technologies.
Pro tip: Develop standardized risk assessment templates for common technology types (cloud platforms, IoT sensors, AI systems) that can be customized for specific implementations. This approach streamlines compliance evaluation while ensuring thoroughness.
Step 2: Define Validation Requirements
Validation demonstrates that your technology can consistently produce results meeting predetermined specifications under normal operating conditions. ISO standards require validation for processes that cannot be fully verified through subsequent inspection or testing—which includes many automated and AI-driven systems.
Develop validation protocols that address:
- Performance specifications: What outputs must the technology deliver?
- Acceptance criteria: How will you determine if performance is adequate?
- Test scenarios: What operating conditions must be validated?
- Sample sizes: How much data is needed to demonstrate consistency?
- Revalidation triggers: What changes require repeating validation?
For complex technologies like AI systems or digital twins, validation may require extensive testing across various operating conditions to demonstrate reliability. Document both your validation approach and the results obtained to create a comprehensive compliance record.
Step 3: Establish Change Control Procedures
Change control ensures that modifications to technology systems are evaluated, approved, documented, and verified before implementation. This is critical for maintaining ISO compliance as technologies evolve through software updates, configuration changes, or capability expansions.
Effective change control procedures should:
- Define what constitutes a “change” requiring formal evaluation
- Establish approval authorities for different types of changes
- Require impact assessment for quality, security, and compliance implications
- Document the rationale for changes and any validation performed
- Maintain version control and configuration management
- Provide rollback procedures if changes cause problems
For cloud-based systems and continuously updated software, work with your vendors to understand their change management processes and ensure you maintain appropriate oversight of changes that could affect quality or compliance.
Step 4: Revise Documentation and Procedures
Implementing new technologies requires comprehensive updates to your existing documentation ecosystem. Start by identifying all procedures, work instructions, and forms that will be affected by the technology change. This documentation revision isn't merely administrative—it's a critical control point that ensures your quality system evolves alongside technological advancements while maintaining ISO compliance.
Documentation updates typically include:
- Process flowcharts showing technology integration points
- Work instructions for operating new systems
- Calibration and maintenance procedures for new equipment
- Data handling and security protocols
- Emergency response procedures for system failures
- Validation and verification procedures
Modern documentation approaches leverage digital systems that can adapt quickly to technological changes. Consider implementing electronic document management systems that support version control, approval workflows, and integration with your production technologies. Leading manufacturers have found that cloud-based documentation systems can reduce document update cycles by up to 70% while improving accessibility for distributed teams managing complex technology implementations.
Step 5: Train Your Team Comprehensively
Even the most sophisticated technologies require properly trained personnel to maintain ISO compliance. Develop comprehensive training programs that address both the technical aspects of new systems and their implications for quality processes. Effective training programs should be role-specific, with different content for operators, maintenance personnel, quality staff, and management to ensure everyone understands their responsibilities in maintaining compliance. Enroll in our QMS Kick-off and Strategic Planning Course
Essential training components:
- Operators: System operation, normal procedures, troubleshooting basics
- Maintenance personnel: Preventive maintenance, calibration, system diagnostics
- Quality staff: Validation verification, audit evidence collection, non-conformance handling
- Management: System oversight, performance monitoring, decision-making protocols
Document all training activities meticulously, as this will be a key focus area during ISO audits of technology implementations. Create a training matrix that maps required competencies against job roles, and establish verification methods to ensure training effectiveness.
Remember that ISO standards emphasize both competence and awareness—personnel must not only know how to operate new technologies but also understand how their actions impact quality outcomes.
Key requirement: Implement both initial and refresher training, particularly when systems change or performance issues are identified. Use a systematic approach to training verification that includes both knowledge assessments and practical demonstrations of competence.
Access our employee overview training →
Digital Transformation: Specific ISO Considerations
Digital transformation introduces unique ISO compliance challenges that extend beyond traditional quality management concerns. As manufacturing operations integrate more digital technologies, the boundaries between quality, information security, and operational technology become increasingly blurred. Forward-thinking manufacturers are adopting integrated management systems that address these interconnected compliance requirements cohesively rather than treating each standard as a separate silo.
Cloud Computing and Data Storage Requirements
Cloud-based manufacturing applications require special attention to both data integrity and security requirements under ISO standards. When migrating quality management processes to cloud platforms, ensure your service providers can demonstrate appropriate controls for data backup, disaster recovery, and access management.
Critical compliance considerations for cloud computing:
Data Sovereignty and Location Understand where your quality data is physically stored and whether this complies with applicable regulations. Some industries or regions have specific requirements about data location that affect cloud provider selection.
Service Provider Certifications ISO auditors increasingly request evidence of cloud service provider certifications (such as SOC 2 or ISO 27001) as part of your overall compliance documentation. Ensure your providers can supply current certification documentation and audit reports.
Access Controls and Authentication Implement robust access management that ensures only authorized personnel can view or modify quality records. Multi-factor authentication, role-based access controls, and audit logging are essential elements.
Data Backup and Recovery Maintain documented backup procedures and test your ability to recover data. ISO standards require that quality records remain accessible and protected from loss—cloud systems must demonstrate these capabilities convincingly.
Contract and SLA Requirements Your agreements with cloud providers should explicitly address data ownership, portability, security requirements, and compliance obligations. These contracts become part of your ISO compliance documentation.
IoT and Connected Factory Equipment
“The integration of IoT technologies with ISO-compliant quality systems represents one of the most significant opportunities for manufacturing advancement in decades. Manufacturers who successfully navigate this integration are seeing dramatic improvements in both quality outcomes and operational efficiency.” — Industry 4.0 Implementation Specialist
Internet of Things (IoT) devices present both opportunities and challenges for ISO compliance. On one hand, these connected sensors can automatically capture quality data that previously required manual recording, reducing human error and improving data accuracy. On the other hand, they introduce new validation requirements to ensure the integrity and security of automated data collection systems.
IoT compliance requirements:
Calibration and Verification Establish clear calibration and verification procedures that meet ISO 9001 requirements for monitoring and measuring equipment. IoT sensors used for quality-critical measurements require the same traceability to recognized standards as traditional measurement devices.
Data Integrity and Validation Document the validation process for any data transformations that occur between sensor collection and your quality management system. This documentation is essential during ISO audits to demonstrate the integrity of your automated data collection processes.
Network Security IoT devices create potential cybersecurity vulnerabilities that must be addressed under ISO 27001 requirements. Implement network segmentation, encryption, and monitoring to protect connected manufacturing systems from cyber threats.
Supplier and Outsourced Process Controls For IoT implementations that span multiple facilities or integrate with supplier systems, pay particular attention to ISO requirements for outsourced processes and supplier controls. Develop clear protocols for handling data from external sources, including validation methods and responsibility assignments.
Blockchain for Supply Chain Integrity Leading manufacturers are implementing blockchain technologies to maintain verifiable audit trails for quality data across complex supply networks while satisfying ISO documentation requirements. This technology provides immutable records of transactions and data exchanges across multi-tier supply chains.
AI and Machine Learning Applications
Artificial intelligence and machine learning systems present unique challenges for ISO compliance due to their inherent complexity and sometimes opaque decision-making processes. When implementing AI in quality-critical applications, ensure you can explain and document the system's decision parameters and validation methods. ISO auditors increasingly expect manufacturers to demonstrate that AI systems are appropriately validated, particularly when they replace human decision-making in quality-critical processes.
Essential AI compliance practices:
Document Training Data Sources Maintain comprehensive records of what data was used to train machine learning models, including data quality verification, bias assessment, and representativeness evaluation. The quality of training data directly impacts AI system reliability.
Establish Algorithm Validation Methods Develop validation protocols that demonstrate AI systems consistently produce correct results across a range of operating conditions. This typically requires extensive testing using diverse data sets that represent real-world variability.
Implement Change Control for Model Updates AI models often require periodic retraining or updates to maintain accuracy. Establish specific change control processes for AI model updates that include impact assessment, validation, and approval before deployment.
Maintain Human Oversight Capabilities Preserve human oversight capabilities for critical quality decisions, even when automated. ISO standards emphasize the importance of human judgment in quality management—ensure AI augments rather than replaces human expertise inappropriately.
Create Comprehensive Audit Trails Implement systems that capture both AI decisions and their underlying rationale. These audit trails are essential for demonstrating compliance during ISO audits and for investigating quality issues when they occur.
Address AI Drift and Monitoring Develop ongoing monitoring approaches that detect when AI model performance degrades over time. Document both your initial validation and ongoing monitoring approach, including how you detect and address drift in AI model performance.
The upcoming 2026 revision of ISO 9001 is expected to address AI governance more explicitly, but manufacturers shouldn't wait to establish appropriate controls. Early adopters are already developing AI governance frameworks that satisfy current ISO requirements while positioning their organizations for compliance with future standards.
Overcoming Common ISO Compliance Challenges When Adopting Technology
Technology adoption introduces several recurring compliance challenges that manufacturers must navigate to maintain ISO certification. Understanding these common pitfalls allows organizations to develop proactive strategies that maintain compliance while still capturing the full benefits of technological innovation. The most successful manufacturers address these challenges through integrated approaches that balance compliance requirements with operational needs.
Challenge 1: Balancing Innovation Speed with Compliance Requirements
The Problem: The tension between rapid innovation and methodical compliance processes represents perhaps the most significant challenge for ISO-certified manufacturers. Technology vendors often push for quick deployments, while thorough compliance evaluation requires careful analysis and documentation.
The Solution: Address this challenge by incorporating compliance considerations into your technology evaluation process rather than treating them as separate workstreams. Develop standardized risk assessment templates for common technologies that streamline the compliance evaluation process while still ensuring thorough analysis.
Best practices:
- Create a cross-functional technology evaluation team including quality, IT, operations, and compliance personnel
- Develop pre-approved technology categories with expedited approval processes
- Establish clear decision criteria that balance innovation benefits against compliance risks
- Implement agile documentation approaches that capture essential compliance information without excessive bureaucracy
- Use pilot implementations to validate compliance approaches before full deployment
This approach allows you to maintain ISO compliance without unnecessarily slowing innovation initiatives. Manufacturers using this structured-yet-flexible approach report 40-50% faster technology implementation timelines compared to traditional sequential approaches.
Challenge 2: Managing Data Security Across Connected Systems
The Problem: As manufacturing systems become increasingly connected, data security emerges as a critical compliance concern that spans multiple ISO standards. A single cybersecurity incident can compromise quality data integrity, disrupt operations, and jeopardize ISO certification.
The Solution: Implement a comprehensive data classification system that identifies quality-critical information assets and applies appropriate security controls based on their sensitivity and compliance implications. Integrate your information security management system (ISMS) with your quality management system (QMS) to ensure consistent security practices across all manufacturing technologies.
Implementation steps:
- Classify data: Identify what data is quality-critical, confidential, or sensitive
- Assess vulnerabilities: Evaluate security risks for each technology component
- Implement layered security: Use multiple defensive measures (network segmentation, encryption, access controls)
- Monitor continuously: Establish security monitoring for connected systems
- Plan incident response: Develop and test procedures for security incidents
- Train personnel: Ensure staff understand security responsibilities
Pay particular attention to technologies that handle quality data or control critical processes. These systems require the highest levels of security controls and monitoring.
Challenge 3: Ensuring Proper Calibration of Advanced Equipment
The Problem: Advanced manufacturing equipment often includes integrated measurement systems that require specialized calibration approaches to satisfy ISO requirements. Traditional calibration methods may not apply to sophisticated sensors, AI-driven inspection systems, or digital measurement technologies.
The Solution: Develop detailed calibration protocols for each technology type, ensuring traceability to recognized standards where applicable. For complex systems where traditional calibration may not be feasible, implement alternative verification methods such as comparative analysis or process capability studies that demonstrate measurement reliability.
Calibration strategies for modern technologies:
IoT Sensors and Smart Instruments Establish calibration intervals based on manufacturer recommendations and operational experience. Implement automated calibration reminders and tracking systems that prevent use of out-of-calibration equipment.
AI-Powered Inspection Systems Develop verification protocols using known-good and known-defective samples that demonstrate the system's ability to consistently make correct determinations. This typically requires extensive testing across various operating conditions and periodic revalidation to ensure continued accuracy.
Digital Measurement Systems Validate digital measurement systems through comparison with traceable reference standards. Document your validation approach and the system's performance over time to create a comprehensive compliance record.
Vision Systems and Automated Inspection Establish performance verification procedures using calibrated test pieces or reference standards. Regular verification ensures these systems maintain accuracy despite changing environmental conditions or component aging.
Document these approaches thoroughly, including your rationale for any non-standard verification methods, to satisfy ISO auditor expectations. Auditors increasingly accept alternative validation methods when they're well-documented and demonstrably effective.
Challenge 4: Maintaining Documentation Currency
The Problem: New technologies often evolve rapidly through software updates, feature additions, and capability expansions. Keeping documentation synchronized with these changes becomes a significant challenge, yet outdated documentation represents a compliance risk.
The Solution: Implement dynamic documentation systems that can be updated quickly without extensive approval cycles for minor changes. Distinguish between substantive changes requiring formal approval and minor updates that can follow streamlined processes.
Documentation management strategies:
- Use version-controlled electronic documentation systems
- Establish clear criteria for when changes require formal approval
- Implement automated change notifications to affected personnel
- Create living documents for rapidly evolving technologies with formal review cycles
- Link documentation directly to training systems to ensure personnel access current information
Challenge 5: Demonstrating Validation for “Black Box” AI Systems
The Problem: Some AI systems, particularly deep learning algorithms, operate as “black boxes” where the decision-making process isn't fully transparent or explainable. This creates challenges for demonstrating validation to ISO auditors who expect clear cause-and-effect relationships.
The Solution: Focus validation efforts on demonstrating that the AI system consistently produces acceptable results across representative operating conditions, even if the internal decision process isn't fully explainable. This outcome-based validation approach satisfies ISO requirements while accommodating AI system complexity.
Validation approach:
- Test extensively with diverse, representative data sets
- Document acceptance criteria and test results comprehensively
- Implement ongoing performance monitoring with statistical process control
- Establish clear protocols for handling anomalous outputs
- Maintain human review capabilities for critical decisions
- Create audit trails that capture inputs, outputs, and confidence levels
Learn more about overcoming innovation paralysis →
Real-World Success Stories: Manufacturers Who Got It Right
Learning from manufacturers who have successfully navigated the dual challenges of technology adoption and ISO compliance provides valuable insights for your own implementation journey. These case studies demonstrate that with proper planning and execution, ISO standards can actually accelerate rather than hinder technological advancement.
Case Study 1: Aerospace Component Manufacturer Implements Digital Twin Technology
Company Profile: Leading aerospace components manufacturer with AS9100 certification (which incorporates ISO 9001 requirements), operating multiple facilities globally producing critical flight components.
Challenge: The company needed to reduce physical prototype testing cycles while maintaining stringent quality standards and regulatory compliance. Traditional testing approaches were time-consuming and expensive, limiting their ability to respond quickly to customer design changes.
Solution Approach: The manufacturer implemented digital twin technology with a validation-first strategy:
Phase 1: Validation Protocol Development Developed comprehensive validation protocols demonstrating equivalence between physical and digital testing methods. The validation approach included:
- Parallel testing (physical and digital) on 50+ component designs
- Statistical analysis demonstrating correlation between simulation and physical results
- Identification of operating boundaries where digital twins were reliable
- Documentation framework mapping digital simulation parameters to physical test requirements
Phase 2: QMS Integration Integrated digital twin platform with existing quality management system:
- Automated data flows from simulations to quality records
- Automatic triggering of simulation refinements when production anomalies occurred
- Real-time validation status dashboards for quality personnel
- Traceability links between digital simulations and physical production lots
Phase 3: Change Management Comprehensive change management including:
- Training programs for engineers, quality staff, and auditors
- Updated procedures and work instructions
- Risk assessments for digital twin limitations
- Fallback procedures for situations where physical testing remained necessary
Results:
- 37% reduction in physical prototype testing cycles
- Zero major findings during AS9100 recertification audit
- 6-month reduction in time-to-market for new component designs
- $2.3M annual savings in prototype material and testing costs
- Maintained full regulatory compliance across all global facilities
Key Success Factor: “We treated digital twin validation like any other process validation—comprehensive, documented, and focused on demonstrating consistent results. This approach gave our auditors confidence that we were maintaining quality standards while embracing innovation.” — Quality Director
Case Study 2: Auto Parts Supplier Transitions to Smart Factory Operations
Company Profile: Tier-1 automotive supplier with IATF 16949 certification, producing safety-critical braking system components for major automotive OEMs across North America.
Challenge: Increasing customer demands for real-time quality data and traceability, combined with pressure to reduce costs and improve efficiency. Legacy systems were disconnected, creating manual data entry burdens and limiting visibility into production performance.
Solution Approach: Comprehensive smart factory implementation with incremental deployment:
Technology Stack:
- IoT sensors on all critical production equipment
- Cloud-based quality management system
- Real-time statistical process control with automated alerts
- Automated data collection eliminating manual quality logs
- Integration with customer portals for real-time quality data sharing
Implementation Strategy: Incremental deployment approach:
- Started with single production line as proof-of-concept
- Validated each technology component before expanding
- Developed controls and documentation incrementally
- Captured lessons learned and refined approach for subsequent lines
Governance Structure: Created cross-functional governance team including:
- Quality management (ensuring ISO/IATF compliance)
- IT (managing cybersecurity and system integration)
- Operations (optimizing production processes)
- Compliance (coordinating audit readiness)
This team developed standardized risk assessment templates for smart factory technologies, streamlining compliance evaluation for each implementation phase.
Results:
- 92% reduction in manual documentation errors
- Full IATF 16949 recertification with zero major findings
- Real-time quality data sharing with automotive OEM customers
- 23% improvement in first-pass yield through faster defect detection
- 15% reduction in quality inspection labor costs
- Enhanced customer satisfaction through improved transparency
Key Success Factor: “Our cross-functional governance team was critical. By bringing quality, IT, operations, and compliance together from day one, we avoided the common pitfall of implementing technology first and addressing compliance later.” — Manufacturing Director
Case Study 3: Medical Device Manufacturer Adopts 3D Printing Technology
Company Profile: Medical device manufacturer with ISO 13485 certification, producing custom orthopedic implants and surgical instruments serving hospitals globally.
Challenge: Need to offer more customized implants while maintaining strict regulatory compliance and lot traceability. Traditional manufacturing methods limited customization options and had long lead times for custom designs.
Solution Approach: Introduced additive manufacturing (3D printing) for critical medical device components through rigorous validation:
Validation Program: Comprehensive process validation demonstrating ability of additive manufacturing to consistently produce components meeting predetermined specifications:
- Material qualification: Extensive testing of biocompatible materials including mechanical properties, biocompatibility, and sterilization compatibility
- Process parameter optimization: Design of experiments identifying critical parameters (layer thickness, print speed, temperature) and acceptable ranges
- Statistical validation: Production of 200+ validation units demonstrating process capability indices exceeding regulatory requirements
- Non-destructive testing validation: Qualification of CT scanning and other inspection methods for detecting internal defects
Quality System Integration:
- Updated device master records with additive manufacturing specifications
- Developed calibration procedures for 3D printing equipment
- Established verification protocols for digital design files
- Created training programs for operators and quality personnel
- Implemented digital traceability from design file to implanted device
Regulatory Strategy:
- Engaged regulatory consultants familiar with additive manufacturing requirements
- Coordinated closely with notified body throughout implementation
- Submitted comprehensive technical documentation demonstrating equivalence to traditionally manufactured devices
- Maintained parallel manufacturing capability during regulatory clearance process
Results:
- ISO 13485 compliance maintained throughout implementation
- Regulatory clearance achieved for additively manufactured devices in 3 markets
- 40% reduction in lead time for custom implant orders
- Enhanced patient outcomes through improved implant customization
- New revenue streams from previously unfeasible custom designs
- Competitive differentiation in increasingly commoditized market
Key Success Factor: “We approached 3D printing implementation exactly like we would any manufacturing process change—with thorough validation, comprehensive documentation, and conservative progression from qualification through production. This methodical approach maintained our ISO 13485 certification while enabling truly innovative patient solutions.” — Regulatory Affairs Manager
Preparing for ISO Audits with New Technology
ISO audits for technology-enhanced manufacturing operations focus heavily on verification that digital systems meet quality requirements consistently. Auditors examine both the technologies themselves and how they're integrated into your broader quality management system. Understanding the auditor's perspective allows manufacturers to prepare effectively and demonstrate compliance confidently.
Prepare for increased scrutiny around system validation during audits after implementing new technologies. ISO auditors will expect to see comprehensive evidence that your technologies consistently produce results meeting predetermined specifications under normal operating conditions. This evidence should include both initial validation documentation and ongoing monitoring data showing continued system performance.
What Auditors Look For in Technology Implementations
ISO auditors typically focus on three key areas when examining technology implementations: process integrity, data integrity, and change control. They want to verify that your digital systems maintain the same level of process control as traditional methods, that data flowing through these systems remains accurate and secure, and that changes to digital configurations are properly managed.
Process Integrity Questions Auditors Ask:
- How does this technology impact your ability to meet product specifications?
- What controls ensure the technology performs consistently?
- How do you detect when the technology isn't performing correctly?
- What happens when the technology fails? Do you have fallback procedures?
- How do you verify that automated processes are working correctly?
Data Integrity Questions Auditors Ask:
- How do you ensure data from this system is accurate?
- What prevents unauthorized modification of quality records?
- How is data backed up and protected from loss?
- Can you demonstrate traceability from raw data to quality records?
- How do you validate data transformations or calculations?
Change Control Questions Auditors Ask:
- How do you manage software updates and configuration changes?
- What approval is required before implementing changes?
- How do you assess the impact of changes on quality?
- Where is change history documented?
- How do you verify changes didn't negatively impact quality?
Be prepared to demonstrate how your systems address these concerns through both documentation and practical demonstrations during the audit. The most successful audit outcomes occur when manufacturers can show both the “paper trail” and the actual system working correctly.
Documentation Requirements for New Technologies
Documentation requirements for new technologies extend beyond traditional quality records to include system specifications, validation protocols, risk assessments, and user training materials. Auditors expect to see clear traceability between system requirements, implemented features, and validation evidence demonstrating that each requirement has been met.
Essential documentation for technology audits:
System Specifications and Requirements
- Functional requirements defining what the system must do
- Technical specifications detailing how requirements are met
- Integration points with existing quality systems
- Performance criteria and acceptance limits
- Security and access control requirements
Validation Documentation
- Validation protocols defining test approach and acceptance criteria
- Test results demonstrating system performance
- Deviation reports and their resolution
- Validation summary report with conclusions
- Revalidation schedule and triggers
Risk Assessments
- Identification of quality, security, and compliance risks
- Probability and severity ratings
- Mitigation strategies for significant risks
- Residual risk assessment after mitigations
- Risk review and update history
Standard Operating Procedures
- System operation procedures for different user roles
- Troubleshooting and error resolution procedures
- Backup and disaster recovery procedures
- Calibration and maintenance procedures (where applicable)
- Emergency procedures for system failures
Training Records
- Training materials covering both operation and compliance aspects
- Training completion records for all users
- Competency assessment results
- Refresher training schedules
- Training effectiveness evaluation
Change Control Records
- Change requests with justification and impact assessment
- Approval documentation
- Implementation verification
- Communication to affected personnel
- Change history and version control
For AI systems and other advanced technologies, additional documentation may be required to explain algorithm operations, data training methods, and decision verification procedures. While ISO standards don't yet provide explicit requirements for AI documentation, forward-thinking manufacturers are developing comprehensive documentation frameworks that address both current standards and anticipated future requirements.
Enroll in our Internal Audit 2 day Online course →
Demonstrating Compliance Through Data and Evidence
The most effective way to demonstrate ISO compliance for new technologies is through comprehensive data collection and analysis that shows system performance over time. Implement monitoring systems that automatically capture key performance indicators related to both process outputs and system operation. These metrics provide powerful evidence during audits that your technologies consistently deliver quality results while meeting ISO requirements.
Key performance indicators to track:
- System uptime and availability
- Process capability indices (Cp, Cpk)
- Defect rates and quality metrics
- Calibration and maintenance completion rates
- User training completion percentages
- Change implementation success rates
- Security incidents and response times
When possible, leverage the technologies themselves to generate compliance evidence—for example, using IoT systems to automatically record calibration verification or process control data. This automated evidence collection reduces manual effort while providing auditors with comprehensive, objective performance data.
Audit presentation best practices:
- Prepare executive summary dashboards showing key compliance metrics
- Have detailed data readily accessible to answer specific questions
- Demonstrate live system operation during the audit
- Show both normal operation and exception handling
- Present trend data showing continuous improvement
- Highlight how technology has enhanced quality outcomes
Remember that auditors appreciate transparency. If you've experienced issues with technology implementation, document how you identified, investigated, and resolved them. This demonstrates mature quality management and continuous improvement—key principles of ISO standards.
Future-Proofing Your Quality Management System
As manufacturing technology continues to evolve rapidly, organizations must develop strategies for maintaining ISO compliance not just today but into the future. This requires building flexibility into quality management systems, creating technology roadmaps aligned with ISO requirements, and fostering an innovation culture that values both advancement and compliance.
Leading manufacturers view future-proofing as a strategic imperative that positions them for sustainable competitive advantage in increasingly complex global markets. Rather than reacting to each new technology individually, these organizations create frameworks that can accommodate innovation systematically while maintaining rigorous quality standards.
Building Flexibility into Your Quality Management System
Create a quality management system that can adapt to technological changes without requiring complete restructuring. Rather than developing rigid procedures tied to specific technologies, focus on outcome-based processes that define what must be achieved rather than exactly how it must be done.
Principles for flexible quality systems:
Outcome-Based Requirements Instead of: “Quality technician shall manually record temperature readings in logbook every 2 hours” Write: “Temperature shall be monitored and recorded at 2-hour intervals with readings traceable to calibrated instruments”
This approach allows you to implement automated IoT temperature monitoring without revising the core procedure—you simply update the work instruction describing the monitoring method.
Modular Documentation Structure Organize your quality system with:
- Policy level: High-level quality policies (rarely change)
- Process level: Core quality processes (change occasionally)
- Procedure level: General procedures (change when processes change)
- Work instruction level: Detailed instructions (change frequently with technology)
This structure allows technology-specific changes at the work instruction level without revising higher-level documentation. Most technology implementations only require updates to work instructions and occasionally procedures.
Technology-Agnostic Language Use terminology that doesn't lock you into specific technologies:
- “Quality management system” rather than “paper-based quality system”
- “Electronic records” rather than “Excel spreadsheets”
- “Automated data collection” rather than “manual data entry”
- “Calibrated measurement equipment” rather than “manual gauges”
Built-In Change Management Incorporate change management processes that explicitly accommodate technology evolution:
- Regular quality system reviews (at least annually)
- Technology refresh planning as part of strategic planning
- Standing cross-functional team authorized to evaluate new technologies
- Pre-approved technology categories with expedited approval processes
Creating a Technology Roadmap That Aligns with ISO Requirements
Develop a forward-looking technology roadmap that incorporates compliance considerations from the beginning rather than treating them as afterthoughts. This roadmap should identify not only which technologies you plan to implement but also how they will integrate with your quality management system and what compliance implications they may present.
Elements of an effective technology roadmap:
Current State Assessment (Year 0)
- Inventory existing technologies and their compliance status
- Identify compliance gaps or risks in current systems
- Document technology-related quality issues or limitations
- Assess workforce technology capabilities and training needs
Short-Term Initiatives (Years 1-2)
- High-priority technology implementations addressing current pain points
- Quick wins that demonstrate technology value while building capability
- Foundation technologies enabling future innovations (e.g., cloud infrastructure)
- Compliance remediation for systems with identified gaps
Medium-Term Initiatives (Years 3-5)
- Strategic technology implementations supporting business objectives
- Integration of initially deployed technologies into comprehensive systems
- Advanced capabilities building on foundational technologies
- Preparation for anticipated regulatory changes (e.g., ISO 9001:2026)
Long-Term Vision (Years 5+)
- Transformational technologies that may fundamentally change operations
- Emerging technologies requiring extended evaluation and development
- Infrastructure investments supporting future innovation
- Workforce development for advanced technology management
For each technology initiative, document:
- Business drivers and expected benefits
- ISO standards impacted and compliance approach
- Integration requirements with existing systems
- Validation and verification strategies
- Risk assessment and mitigation plans
- Resource requirements (budget, personnel, time)
- Dependencies on other initiatives
- Key milestones and decision points
By addressing potential compliance challenges early in the planning process, you can avoid costly delays and rework during implementation while ensuring your technology investments deliver their full potential value.
Developing an ISO-Compliant Innovation Culture
Foster an organizational culture that values both innovation and compliance rather than viewing them as competing priorities. This requires educating personnel at all levels about how ISO standards can actually enable rather than restrict technological advancement when properly implemented.
Strategies for building innovation culture:
Leadership Commitment Leaders must consistently communicate that innovation and compliance are complementary, not contradictory. Demonstrate this through:
- Allocating resources for both technology and compliance initiatives
- Celebrating examples where ISO frameworks accelerated innovation
- Addressing compliance obstacles that genuinely hinder innovation
- Including both innovation and compliance metrics in performance evaluations
Cross-Functional Collaboration Create cross-functional teams that bring together technical, operational, and quality perspectives to evaluate new technologies and develop implementation strategies that satisfy both innovation and compliance objectives. These teams break down silos and ensure comprehensive evaluation of technology proposals.
Education and Awareness Implement training programs that help personnel understand:
- How ISO standards provide structure for managing technology change
- Examples of how compliance frameworks have prevented quality issues
- The business value of ISO certification beyond mere compliance
- How to incorporate compliance thinking into technology evaluation
Recognition and Rewards Recognize and reward employees who identify innovative ways to leverage new technologies while maintaining or enhancing compliance. This positive reinforcement helps embed compliance thinking throughout your innovation process rather than treating it as a separate checkpoint.
Examples to celebrate:
- Identifying technology solutions that improve both efficiency and compliance
- Developing innovative validation approaches for new technologies
- Creating reusable compliance frameworks that accelerate future implementations
- Finding ways to reduce compliance burden through automation
Continuous Improvement Mindset Encourage questioning of compliance practices that don't add value. Not all traditional approaches remain optimal as technologies evolve. Create safe channels for personnel to:
- Suggest more efficient compliance approaches
- Identify redundant or outdated requirements
- Propose technology solutions to compliance challenges
- Challenge assumptions about what ISO standards require
Leading manufacturers find that this integrated approach not only improves compliance outcomes but also accelerates technology adoption by reducing implementation barriers and building organizational capability for managing change effectively.
Preparing for ISO 9001:2026 and Beyond
The upcoming 2026 revision of ISO 9001 will explicitly address Industry 4.0 technologies, digital transformation, and emerging manufacturing paradigms. While specific requirements aren't finalized, manufacturers can prepare now by implementing practices aligned with anticipated directions.
Expected areas of focus in ISO 9001:2026:
- AI and machine learning governance frameworks
- Cybersecurity integration with quality management
- Digital documentation and electronic signature standards
- Cloud computing and data integrity requirements
- Automated decision-making validation and oversight
- Supply chain visibility and traceability in digital ecosystems
- Sustainability and environmental considerations in quality management
Preparation strategies:
- Implement AI governance frameworks now, even if not explicitly required
- Strengthen cybersecurity practices and integrate them with quality systems
- Transition to cloud-based systems with appropriate controls and validation
- Establish digital traceability across supply chains and production processes
- Document technology decision-making processes and validation approaches
- Monitor ISO technical committee activities and participate in industry feedback
- Build flexibility into quality systems to accommodate future requirements
Frequently Asked Questions
Do I need to notify my ISO certification body before implementing new technology?
While there's typically no formal requirement to notify your certification body before implementing new technology, it's often advisable for significant changes that substantially impact your quality processes. At minimum, document your change management process thoroughly and be prepared to present this documentation during your next surveillance audit.
For major technology implementations that fundamentally change how you address ISO requirements, consider requesting a pre-assessment from your certification body to identify any potential compliance issues before your formal audit. This proactive approach can prevent surprises during certification audits and provide valuable guidance during implementation.
When to notify your certification body:
- Complete replacement of your quality management system software
- Implementation of fully automated production lines
- Major changes to critical quality processes
- Technologies affecting multiple ISO standard requirements simultaneously
- First-time implementation of emerging technologies (AI, blockchain, etc.)
How often should I update my risk assessment when adopting new manufacturing technologies?
Risk assessments should be updated whenever you implement new technologies that could affect product quality or process performance. This includes initial implementation, significant system updates, and changes to how the technology interfaces with other systems.
Beyond these event-driven updates, establish a regular review cycle—typically quarterly for rapidly evolving technologies and annually at minimum—to ensure your risk assessments remain current as both your technologies and operating environment evolve.
Remember that ISO standards emphasize risk-based thinking throughout your quality management system, not just in formal risk assessment documents. Train personnel to continually evaluate how technology changes might impact quality risks and to initiate formal assessment updates when significant changes occur. This ongoing vigilance helps maintain compliance while preventing quality issues before they emerge.
Triggers for risk assessment updates:
- New technology implementation or pilot programs
- Software updates that change functionality
- Integration of systems that were previously independent
- Changes to supplier or cloud service providers
- Security incidents or near-misses
- Quality issues potentially related to technology
- Regulatory or standard requirement changes
Can ISO standards actually help speed up technology adoption rather than slow it down?
Yes, when properly implemented, ISO standards can accelerate technology adoption by providing structured frameworks for managing change while maintaining quality. The risk-based approach embedded in modern ISO standards helps organizations identify and address potential implementation issues early, reducing costly rework and implementation delays.
Additionally, the process documentation required by ISO standards creates institutional knowledge that supports more efficient technology deployment across multiple facilities or similar applications. Instead of reinventing approaches for each implementation, organizations can leverage documented lessons learned and proven strategies.
How ISO frameworks accelerate technology adoption:
Structured Decision-Making ISO's risk-based thinking provides frameworks for systematically evaluating technologies, eliminating analysis paralysis and endless debate cycles.
Reduced Rework Thorough validation and testing before full deployment prevents costly failures that require starting over. ISO's emphasis on process validation catches issues early.
Reusable Frameworks Documentation created for initial implementations becomes templates for subsequent projects, dramatically reducing effort for similar technologies.
Stakeholder Confidence ISO compliance provides assurance to leadership, customers, and regulators that innovations maintain quality standards, accelerating approval processes.
Continuous Improvement Focus ISO's emphasis on monitoring and improvement creates feedback loops that rapidly optimize technology performance after deployment.
Leading manufacturers leverage ISO frameworks to create standardized technology evaluation and implementation processes that can be applied consistently across their operations. This standardization eliminates redundant work, captures lessons learned, and builds organizational capability for managing technological change effectively.
“When we aligned our digital transformation strategy with our ISO quality framework, we not only maintained compliance but actually accelerated our technology implementation timeline by eliminating rework and focusing resources on critical success factors. Our ISO system wasn't a roadblock—it was a roadmap.” — Manufacturing Technology Director
Learn how to overcome innovation paralysis →
What documentation is essential when implementing AI or automation in ISO-certified manufacturing?
Essential documentation for AI and automation implementations in ISO-certified environments includes system specifications, validation protocols, risk assessments, algorithm descriptions, data governance procedures, and user training materials.
Critical documentation elements:
System Specifications
- Functional requirements defining intended use
- Performance criteria and acceptance limits
- Integration points with quality systems
- Security and access control requirements
- Limitations and boundaries of use
Validation Documentation
- Validation protocol with test approach
- Training data sources and quality verification (for AI)
- Test results across normal and boundary conditions
- Performance metrics and statistical analysis
- Validation summary and conclusions
- Ongoing monitoring approach
Algorithm Documentation (AI-Specific)
- High-level description of algorithm approach
- Training data selection and validation methods
- Key parameters and decision thresholds
- Known limitations or biases
- Version control and change history
Data Governance
- Data classification and handling requirements
- Access controls and security measures
- Backup and recovery procedures
- Data retention and archival policies
- Privacy and confidentiality considerations
Risk Assessment
- Potential failure modes and effects
- Probability and severity ratings
- Mitigation strategies
- Residual risk evaluation
- Monitoring and review procedures
Operating Procedures
- Standard operating procedures for different user roles
- Troubleshooting and error handling
- Override procedures and escalation paths
- Maintenance and calibration (where applicable)
- Emergency response for system failures
Training Materials
- User training covering operation and compliance
- Competency assessment methods
- Training completion records
- Refresher training schedules
For AI systems specifically, documentation should address how training data was selected and validated, how algorithm performance is monitored, and what controls ensure the system makes appropriate decisions in all operating conditions. Maintain comprehensive records of testing results that demonstrate system performance across normal, boundary, and exception conditions to satisfy both current and anticipated ISO requirements.
How do I train employees on both new technology and maintaining ISO compliance?
Effective training programs integrate technology operation and compliance requirements rather than treating them as separate topics. Develop role-specific training that explains not just how to use new technologies but why specific procedures matter for compliance and quality outcomes.
Comprehensive training approach:
Role-Specific Content
- Operators: System operation, normal procedures, basic troubleshooting, quality impact of their actions
- Maintenance Personnel: Preventive maintenance, calibration, diagnostics, system integrity verification
- Quality Staff: Validation verification, audit evidence collection, non-conformance handling, monitoring approaches
- Management: System oversight, performance monitoring, decision authorities, escalation procedures
Integrated Learning Objectives Design training that addresses:
- Technical operation of the technology
- Why procedures exist and how they protect quality
- Compliance requirements and audit expectations
- Problem recognition and escalation
- Documentation responsibilities
- Security and access control practices
Practical Application Use hands-on exercises and practical examples that demonstrate the connection between proper technology usage and ISO requirements. This integrated approach helps employees understand both the “how” and the “why” behind compliance procedures, improving adherence and problem-solving capabilities.
Technology-Enhanced Training Leverage technology itself to enhance training effectiveness:
- Learning management systems for tracking completion and competency
- Augmented reality tools providing real-time guidance on compliant process execution
- Simulation environments for practicing without production impact
- Digital job aids accessible at point of use
- Video demonstrations showing correct procedures
Verification and Documentation Implement systematic training verification including:
- Knowledge assessments (written tests or oral examinations)
- Practical demonstrations of competence
- On-the-job evaluation during initial operations
- Documentation of training completion, assessment results, and competency determination
Ongoing Support Establish coaching and support mechanisms that help employees navigate compliance requirements as they become more proficient:
- Accessible subject matter experts
- Clear escalation paths for unusual situations
- Regular refresher training sessions
- Performance feedback and correction
- Updates when systems change or issues are identified
Remember that ISO standards require both initial and refresher training, particularly when systems change or performance issues are identified. Build this into your training program design rather than treating it as an afterthought.
Access our technology training program template →
What should I do if my technology vendor's system doesn't fully meet ISO requirements?
This is a common challenge that requires careful evaluation and risk-based decision-making. The key is understanding which requirements are truly essential versus which represent your preferred approach, and then determining whether gaps can be mitigated through complementary controls.
Systematic gap analysis approach:
1. Identify Specific Gaps Document precisely which ISO requirements aren't met by the vendor's system. Be specific—”doesn't meet audit trail requirements” is too vague, while “doesn't capture user identity for record modifications” identifies a specific gap.
2. Assess Risk and Impact Evaluate the risk associated with each gap:
- Critical gaps: Directly affect product quality or regulatory compliance
- Significant gaps: Create compliance risk or operational challenges
- Minor gaps: Represent preferences rather than requirements
3. Explore Mitigation Options For each gap, identify potential solutions:
- Vendor configuration: Can settings or modules address the gap?
- Vendor development: Will the vendor add capabilities (timeline? cost?)?
- Complementary controls: Can you address gaps through procedures or additional systems?
- Alternative approaches: Can you meet the intent differently than planned?
4. Make Risk-Based Decisions ISO standards don't dictate specific technology solutions—they require managing quality risks effectively. If you can demonstrate that your overall approach (vendor system plus mitigations) maintains quality and traceability, you can achieve compliance even if the system alone doesn't meet every preference.
Example mitigation strategies:
- Gap: System doesn't prevent use of out-of-calibration equipment
- Mitigation: Implement procedural controls with periodic verification audits
- Gap: System lacks certain statistical analysis capabilities
- Mitigation: Export data to validated analysis tools for required calculations
- Gap: Audit trail doesn't capture specific information
- Mitigation: Supplement with manual logs for critical activities
5. Document Your Approach Create comprehensive documentation showing:
- Requirements that must be met and why
- How the vendor system addresses each requirement
- Identified gaps and their risk assessment
- Mitigation strategies for each gap
- Validation that mitigated approach maintains compliance
This documented, risk-based approach demonstrates mature quality management and typically satisfies ISO auditors even when technology doesn't perfectly align with every requirement.
Conclusion: Your Next Steps for ISO-Compliant Technology Adoption
Maintaining ISO compliance while adopting new manufacturing technologies isn't just possible—it's a strategic advantage that forward-thinking manufacturers are leveraging to accelerate innovation, improve quality outcomes, and strengthen competitive positioning.
Key Takeaways
ISO Standards Enable Innovation Rather than hindering progress, properly implemented ISO frameworks provide structured approaches for managing technological change while maintaining quality. Manufacturers who integrate compliance considerations early in technology evaluation see faster implementations and better outcomes.
Systematic Approaches Deliver Results The five-step framework—risk assessment, validation definition, change control, documentation revision, and comprehensive training—provides a proven roadmap for implementing technologies while maintaining certification.
Integration Is Essential Modern manufacturing technologies impact quality, security, environmental, and safety considerations simultaneously. Integrated management systems that address multiple ISO standards cohesively are more efficient and effective than siloed approaches.
Preparation Pays Dividends With ISO 9001:2026 explicitly addressing Industry 4.0 technologies, manufacturers who build flexible quality systems and establish technology governance frameworks now will be well-positioned for future requirements.
Documentation Creates Value Comprehensive documentation of technology implementations creates institutional knowledge that supports both compliance and continuous improvement, accelerating future technology adoptions.
Your Action Plan
Immediate Actions (This Week)
- Assess your current state: Inventory technologies already implemented or planned
- Identify compliance gaps: Where might current approaches fall short of ISO requirements?
- Establish governance: Create or empower a cross-functional team to oversee technology adoption
- Review this guide: Share with stakeholders involved in technology decisions
Short-Term Actions (This Month) 5. Develop risk assessment templates: Create standardized frameworks for evaluating common technologies 6. Update your technology roadmap: Incorporate compliance considerations into technology planning 7. Audit your documentation: Ensure existing technology implementations are adequately documented 8. Assess training needs: Identify gaps in personnel competency for current and planned technologies
Ongoing Actions (This Quarter and Beyond) 9. Implement flexible quality systems: Transition to outcome-based procedures that accommodate technology evolution 10. Build innovation culture: Foster environments where compliance and innovation coexist 11. Monitor ISO developments: Stay informed about ISO 9001:2026 and other standard updates 12. Capture lessons learned: Document experiences to accelerate future implementations
How MSI Can Help
Navigating the intersection of technology adoption and ISO compliance requires deep expertise in both domains. MSI specializes in helping manufacturing companies embrace digital transformation while maintaining rigorous quality standards.
Our services include:
- ISO compliance consulting for manufacturers implementing new technologies
- Technology readiness assessments evaluating compliance implications before implementation
- Validation protocol development for AI, IoT, cloud systems, and other advanced technologies
- Gap analysis and remediation for existing technology implementations
- Audit preparation support ensuring you're ready to demonstrate compliance confidently
- Training program development integrating technology operation with compliance requirements
- Integrated management system design addressing multiple ISO standards cohesively
With experience across aerospace, automotive, medical device, electronics, and other highly regulated industries, MSI understands the unique challenges manufacturers face when balancing innovation with compliance.
Ready to Get Started?
Don't let ISO compliance concerns slow your technology adoption or, worse, cause you to avoid beneficial innovations altogether. With the right approach and expert guidance, you can maintain certification while leveraging technologies that transform your operations.
Take the next step:
- Schedule a free consultation to discuss your specific technology adoption challenges
- Evaluate your current compliance posture
- Access our resources for templates, checklists, and additional guidance
The future of manufacturing is digital, connected, and intelligent. Ensure your organization is positioned to capture these opportunities while maintaining the quality standards that protect your reputation and market access.
Contact MSI today to learn how we can support your journey toward ISO-compliant digital transformation.
Last updated: October 2025
About the Author: This guide was developed by MSI's team of ISO compliance experts and manufacturing technology specialists, drawing on decades of combined experience helping manufacturers navigate complex regulatory requirements while embracing innovation.
Related Resources:
- Understanding FDA QMM for ISO-Certified Companies
- ISO Standards for Innovation & Expansion Support
- How to Overcome Innovation Paralysis in Manufacturing
- Management Systems: The Foundation of Business Resilience and Growth

