Contents
- Steer Your Medical Device Quality to Success
- Set the Course: Understanding Quality Management Systems
- On the Right Track: Developing and Implementing Your QMS
- Avoiding Rough Waters: Risk Management and Compliance
- Safeguarding the Journey: Control Measures and Auditing
- Navigational Aids: Software and Tools for Quality Management
- Arriving at the Destination: Maintaining and Improving Your QMS
- Set Sail Toward Quality Mastery
- Why Continuous Learning is Your Ultimate Compass in Quality Management
- Upgrade Your QMS Knowledge with This Comprehensive Guide
- Chart New Territories: Advanced Quality Management Techniques
- FAQ
- What Are the First Steps to Implement a QMS for Medical Devices?
- How Often Should a Medical Device QMS be Audited?
- What Are the Benefits of Electronic Quality Management Systems?
- How Do ISO Standards Enhance Medical Device Quality?
- Can Small Medical Device Manufacturers Afford QMS Implementation?
Key Takeaways
- ISO 13485 is the cornerstone for a Quality Management System in the medical device industry, ensuring consistent product quality and regulatory compliance.
- Developing a robust QMS requires a clear quality policy, comprehensive process design, and a well-trained team.
- Risk management, as outlined in ISO 14971, is vital for identifying and mitigating potential hazards associated with medical devices.
- Regular audits and control measures are crucial for maintaining QMS effectiveness and readiness for regulatory inspections.
- Continuous improvement and education in quality management are essential for adapting to changes in the medical device industry and maintaining a competitive edge.
Steer Your Medical Device Quality to Success
In the medical device industry, where the stakes include human health and safety, navigating through the complexities of quality management is not just a regulatory requirement; it’s a moral imperative. As professionals in this field, we understand that the journey towards quality excellence is continuous, ever-evolving and improves performance. The cornerstone of this journey is the Quality Management System (QMS), which, when implemented correctly, serves as a compass, guiding your organization towards the pinnacle of product quality and compliance. If your company is ISO 9001 certified know that the current ISO 13485: 2016 is really ISO 9001: 2008 version. So if your company is ISO 9001 certified its easy to add on ISO 13485 requirements. So guess what? Preventive action gets added back in.
A Quick Look at Why Quality Management is Critical
Let’s face it, in the world of medical devices, quality isn’t just a box to tick; it’s the foundation upon which trust is built with patients, practitioners, and regulators. A solid QMS helps ensure that every device that leaves your production line isn’t just good—it’s life-saving good. That’s because a QMS doesn’t just protect users; it shields your company from the stormy seas of non-compliance, which can lead to recalls, legal issues, and damaged reputations. The US Food and Drug Administration (FDA) has recently announced its intention to use ISO 13485 as the basis for its quality system legislation for medical devices. ISO 13485 is an internationally accepted standard that specifies the requirements for a quality management system (QMS) for the medical devices industry. FDA is aligning its requirements to this standard to drive a global convergence of medical device regulatory processes2.
The Roadmap to Quality Excellence
Embarking on the quality management journey requires a roadmap, and that’s where ISO 13485 comes in. In MSI’s roadmap SurePath – Management Systems International (MSI) (msi-international.com) the first activity is to create the program plan to roll out a Quality Management System. It’s not just a set of guidelines; it’s a blueprint for building a QMS that stands up to scrutiny, ensuring that your medical devices are consistently hitting the mark when it comes to quality and safety.
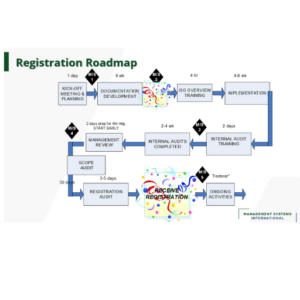
Paths to Streamlining Your Quality Compliance
Beyond just meeting standards, an efficient QMS streamlines your operations, paving the way for innovation and growth. It helps identify process bottlenecks, minimizes waste, and ensures that your products not only meet but exceed customer expectations.
Set the Course: Understanding Quality Management Systems
At the heart of quality management navigation is a deep understanding of what a QMS entails. It’s not just a set of documents or a checklist; it’s a living system that reflects your organization’s commitment to excellence. A well-designed QMS encompasses the entirety of your operations, from design and development to production, distribution, and post-market surveillance.
ISO 13485: The Beacon for Medical Device Quality
ISO 13485 is the international standard for QMS in the medical device industry. It’s the benchmark against which organizations measure their quality processes. Adhering to this standard ensures that your products are designed and manufactured under controlled conditions, consistently meet regulatory requirements, and achieve customer satisfaction.
But why ISO 13485? Because it’s tailored for the medical device sector, focusing on areas such as sterilization, traceability, and product safety which are critical in our field. This standard isn’t just about compliance; it’s about setting up a system that can withstand the test of time and change.
So, if you’re looking to navigate the waters of medical device quality management successfully, aligning with ISO 13485 is your first port of call.
Crafting a Quality Policy that Guides Your Mission
A quality policy is your organization’s declaration of your commitment to quality. It should reflect your company’s unique vision and set the tone for your QMS. Think of it as the North Star for your employees, providing direction and purpose in their daily tasks. Must Haves” that must be addressed in your Quality Policy, includes a commitment to comply with requirements and to maintain the effectiveness of the quality management system.
Process Design: Blueprinting Your Way to Success
Designing your processes with quality in mind is like drafting the blueprints for a sturdy ship. This is the common project/program planning identifying team members of the leadership team, suggested timeline, tasks intended documentation to be developed for the various levels and record/forms to be established. QMS requires careful planning, a deep understanding of your destination (product requirements), and the right materials (resources) to ensure a safe and successful voyage so that the system is sustainable and no major rework later. Common pitfalls companies do is using a documentation numbering system that aligns with the standard, but this is a mistake due to the numbering system can change. Use the completion of your full system internal audits and performance of your Management Review Combo Medical Device ISO 13485 And ISO 9001 Management Review Toolkit – Management Systems International (MSI) (msi-international.com) as major milestones.
On the Right Track: Developing and Implementing Your QMS
Once you have the blueprint, it’s time to build. Developing and implementing a QMS is a team effort. It’s about bringing together the right mix of expertise, from expert ISO Consultant, documentation administrator design engineers to quality control inspectors, and ensuring they work in harmony towards the same goal. We suggest representatives from most departments, but not necessary to include finance.
Team Assembly: Choosing the Right Crew for Quality Management
Your team is the crew that will navigate your QMS. Selecting individuals with the right skills and attitude is crucial. They need to be detail-oriented, process-driven, and, most importantly, committed to the quality ethos that your organization stands for.
Navigation Tools: Essential QMS Software for Medical Devices
In today’s digital age, QMS software has become an indispensable tool. These systems can automate workflows, streamline document control, and provide real-time data for decision-making. Choosing the right QMS software is like picking the best navigation tools; they should enhance your ability to steer through regulatory requirements with ease. Expert information to know that any software used in relations to the QMS and especially documentation control must be verified as being effective. document procedures for the validation of the application of computer software used in the quality management system. Such software applications shall be validated prior to initial use and, as appropriate, after changes to such software or its application”.
Embark on Training: Educating Your Team on QMS Fundamentals
Training your team is not just about compliance; it’s about empowerment. By educating your crew on the fundamentals of your QMS, you’re equipping them with the knowledge to not just follow procedures but to understand the ‘why’ behind them.
- Identify the core elements of your QMS and ensure everyone is familiar with them.
- Use engaging and interactive training methods to reinforce learning.
- Encourage a culture of continuous learning and improvement.
By laying a strong foundation with a well-crafted QMS, you’re setting your organization on a course for success. But remember, a QMS is not a ‘set it and forget it’ system. It requires ongoing attention, regular audits, and a culture of continuous improvement to stay on course.
Avoiding Rough Waters: Risk Management and Compliance
As we navigate the complex seas of medical device manufacturing, risk management stands as the lighthouse, guiding us away from potential dangers. In our industry, the margin for error is narrow, and the consequences can be grave. Therefore, understanding and managing risks is not just a regulatory requirement, but a responsibility we owe to every patient and healthcare professional who relies on our products.
Effective risk management means identifying potential hazards early, assessing their impact, and implementing controls to mitigate them. It’s a proactive approach that not only safeguards patients but also protects the integrity of your business.
ISO 14971: Charting Risks and Safeguarding Health
ISO 14971 is the international standard for the application of risk management to medical devices. It provides a systematic framework for manufacturers to identify hazards associated with medical devices, to estimate and evaluate associated risks, to control these risks, and to monitor the effectiveness of the controls. The goal is to ensure that the benefits of a product outweigh its risks.
Staying Afloat: Strategies for Compliance with FDA Regulations
Navigating FDA regulations requires a keen understanding of the ever-evolving compliance landscape. It’s about staying afloat amidst the tides of regulatory changes and ensuring that your quality management system is robust enough to withstand scrutiny. Staying compliant means being vigilant, conducting regular internal audits, and keeping your documentation in shipshape.
Continuous Monitoring: Ensuring a Smooth Sail Through Quality Assurance
Quality assurance is the compass that keeps us on course, ensuring that our products consistently meet the highest standards of efficacy. Through continuous monitoring of our processes and outcomes, we can detect any deviations and correct our course promptly. It’s a dynamic process that demands our constant attention and commitment.
Safeguarding the Journey: Control Measures and Auditing
The key to maintaining a steady course in quality management is establishing strong control measures. These measures act as the bulwarks that protect the integrity of our medical devices. They range from rigorous testing and validation protocols to thorough documentation practices, all designed to ensure that our products are safe and effective.
Quality Checks: Fortifying the Gates of Device Safety
Quality checks are the checkpoints in our journey towards device safety. They are the moments where we verify that our products are not just meeting, but exceeding, the required standards. These checks are embedded throughout our manufacturing process, from initial design to final delivery, ensuring that every product that leaves our facility is worthy of the trust placed in it.
Moreover, these quality checks are not static. They evolve as new technologies and methods become available, ensuring that our commitment to safety is never compromised.
Preparing for Inspections: Mock Audits and Gap Analyses
To ensure we’re always inspection-ready, we conduct mock audits and gap analyses. These exercises are like dress rehearsals for the real thing, allowing us to identify any areas of non-compliance and address them before they become issues. By simulating the stringent conditions of an FDA inspection, we can build confidence and readiness within our team.
Corrective Actions: Navigating Through Turbulent Non-conformance
When we encounter non-conformance, it’s essential to act swiftly and effectively. Corrective actions are our means of righting the ship, ensuring that any deviations are addressed and rectified. This not only prevents similar issues from occurring in the future but also demonstrates our commitment to continuous improvement.
Navigational Aids: Software and Tools for Quality Management
In the quest for quality, we’re fortunate to have an array of navigational aids at our disposal. Software and tools specifically designed for quality management can streamline our processes, enhance our efficiency, and provide us with the data we need to make informed decisions.
These tools can automate routine tasks, facilitate communication across departments, and provide a centralized repository for all our quality-related documentation. In essence, they are the modern instruments that help us chart a precise course in quality management.
Electronic QMS: Revolutionizing Device Quality Oversight
An Electronic Quality Management System (eQMS) is revolutionizing the way we oversee device quality. It allows for greater visibility into our processes, provides a platform for collaboration, and ensures that our documentation is always up-to-date and accessible. With an eQMS, we’re not just keeping up with the times; we’re setting the pace for the future of quality management.
The benefits of an eQMS include:
- Reduced paperwork and manual errors.
- Improved traceability and accountability.
- Streamlined audit processes.
- Enhanced regulatory compliance.
Adopting an eQMS is not just a smart move; it’s a strategic one that can elevate the quality of our products and the efficiency of our operations.
Tracking and Tracing: Tools for Ensuring Quality Across the Supply Chain
Quality doesn’t start or end at our factory doors; it extends across the entire supply chain. Tracking and tracing tools enable us to monitor our products’ journey from raw materials to finished goods, ensuring that quality is maintained at every step. These tools provide transparency and accountability, key components of a trusted quality management system.
Calibration and Maintenance: Keeping Your Equipment on the Precise Course
The accuracy of our testing and production equipment is paramount. Calibration and maintenance are the routine checks and adjustments we make to ensure that our equipment performs correctly. It’s like tuning the instruments of an orchestra to ensure harmony; each piece must be in perfect working order to achieve the desired outcome.
Arriving at the Destination: Maintaining and Improving Your QMS
The journey towards quality management excellence is ongoing. It doesn’t end with the implementation of a QMS or the achievement of a certification. It’s a continuous process of maintaining and improving our systems to adapt to new challenges and opportunities.
Sustaining Success: Enhancing Your QMS with Lean Principles
Lean principles provide a framework for enhancing our QMS by focusing on value, flow, and eliminating waste. By applying these principles, we can streamline our processes, reduce costs, and improve the overall quality of our products. It’s about doing more with less and doing it better.
Embracing lean is not just about cutting costs; it’s about creating value for our customers and for our organization. It’s a philosophy that aligns perfectly with our commitment to quality and continuous improvement.
Beyond Compliance: Fostering a Culture of Quality Improvement
Finally, the true north of our quality management navigation is the culture we foster within our organization. It’s about going beyond compliance and embedding quality into the DNA of our company. By encouraging innovation, recognizing excellence, and promoting transparency, we create an environment where quality thrives.
And when quality is part of our culture, it becomes more than just a requirement; it becomes our identity. It’s what sets us apart in the marketplace and what ensures our long-term success.
To continue your journey in mastering quality management for medical devices, and to ensure your QMS not only meets but exceeds ISO certification requirements, learn more about the SureResults® program. This comprehensive guide and proactive maintenance program will be your dynamic partner in sustaining and enhancing the performance of your quality or environmental management system.
Beyond Compliance: Fostering a Culture of Quality Improvement
Quality management in the medical device industry is not just about checking boxes to meet regulatory requirements. It’s about embedding a culture of quality that permeates every aspect of your organization. This cultural shift can lead to not only improved products but also to a more engaged and proactive workforce.
Here are some strategies to foster a culture of quality improvement:
- Encourage open communication about quality issues and successes.
- Recognize and reward employees who contribute to quality improvements.
- Provide continuous training and professional development opportunities.
- Involve all levels of the organization in quality initiatives.
Refining Your Compass: Using Feedback for QMS Perfection
Feedback is the wind that propels the ship of quality management forward. It comes from customers, employees, and the quality management system itself. By actively seeking out and responding to feedback, you can identify areas for improvement and refine your QMS to better serve the needs of all stakeholders.
Set Sail Toward Quality Mastery
Quality management navigation is an ongoing journey. It requires commitment, diligence, and a willingness to continuously learn and adapt. The ultimate goal is to not just comply with regulatory standards but to achieve quality mastery—a state where your QMS is a dynamic, integral part of your organization, driving continuous improvement and operational excellence.
Why Continuous Learning is Your Ultimate Compass in Quality Management
Continuous learning is the lifeblood of quality management. In an industry where regulations, technologies, and best practices are always evolving, staying informed is not optional—it’s essential. By committing to ongoing education, you ensure that your QMS remains effective, efficient, and aligned with the latest industry standards.
Whether it’s attending workshops, enrolling in certification courses, or simply keeping up with industry literature, make learning a priority. It’s an investment that pays dividends in the form of better products, smoother operations, and a stronger competitive edge.
Upgrade Your QMS Knowledge with This Comprehensive Guide
Enhancing your knowledge about quality management systems is crucial for staying ahead in the medical device industry. With the right resources, you can deepen your understanding of QMS principles, best practices, and regulatory requirements.
To get started, learn more about the SureResults® program—a proactive maintenance program that will help you maintain and enhance your QMS, ensuring long-term success and profitability.
With SureResults®, your management system not only meets but exceeds ISO certification requirements, adapting to changes and maintaining profitability for long-term success.
Chart New Territories: Advanced Quality Management Techniques
As you become more comfortable with the basics of quality management, it’s time to explore advanced techniques that can further improve your QMS. These might include statistical process control, Six Sigma, and advanced risk assessment methodologies. These techniques can help you gain deeper insights into your processes, identify areas for improvement, and optimize performance.
FAQ
What Are the First Steps to Implement a QMS for Medical Devices?
The first steps to implement a QMS for medical devices involve understanding the specific requirements of the ISO 13485 standard, assembling a dedicated team, and developing a comprehensive quality policy. From there, you’ll need to design and document your processes, provide training, and establish mechanisms for monitoring and continuous improvement.
How Often Should a Medical Device QMS be Audited?
A medical device QMS should be audited at least annually to ensure ongoing compliance with regulatory standards and to identify opportunities for improvement. However, more frequent audits may be necessary depending on the complexity of your processes, changes in regulations, or the occurrence of quality issues. Audit schedules are based on importance of the processes, changes in your organization and previous audit results. Our primary approach is for all requirements to be audited early part of the year then a few months later the requirements your company should audit more frequently. Typically the weak areas are design and development, purchasing, nonconforming product and corrective/preventive action.
What Are the Benefits of Electronic Quality Management Systems?
Electronic Quality Management Systems (eQMS) offer numerous benefits, including improved document control, more efficient process management, enhanced traceability, and better data analysis capabilities. An eQMS can also facilitate compliance with regulatory standards and improve audit readiness.
How Do ISO Standards Enhance Medical Device Quality?
ISO standards, such as ISO 13485, provide a framework for establishing a QMS that ensures consistency in design, development, production, and delivery of medical devices. They help organizations to meet both customer expectations and regulatory requirements, thereby enhancing the overall quality of medical devices.
Can Small Medical Device Manufacturers Afford QMS Implementation?
Yes, small medical device manufacturers can afford QMS implementation. While the initial investment may seem daunting, the long-term benefits of improved efficiency, reduced waste, and enhanced product quality often outweigh the costs. Additionally, there are scalable QMS solutions available that are suitable for smaller operations.
In conclusion, quality management navigation is an essential aspect of the medical device industry, providing the direction needed to ensure products are safe, effective, and compliant with regulatory standards. By implementing a robust QMS, engaging in continuous learning, and utilizing advanced quality management techniques, medical device professionals can steer their organizations toward success. Remember, quality is not a destination but a journey of continuous improvement.